A comprehensive database of GCP quizzes online test your knowledge with GCP quiz questions. According to ICH GCP the sponsor is responsible for appointing monitors.

Society For Clinical Data Management Scdm Linkedin
Basically I will walk you through the entire GCP guidelines document step by step.

. Exam Hall A Level 4 Educational Block Faculty of Medicine UKM Jalan Yaacob Latif 56000 Cheras Kuala Lumpur. Since the inception of the Clinical Research Centre by Ministry of Health in August 2000 Good Clinical Practice GCP has become an important aspect in the conduct of clinical trials in Malaysia. Access over 3000 free questions and 2000 flashcards on our new quiz platform.
Malaysian Guideline for Good Clinical Practice 4th Ed Malaysian Guideline for Good Clinical Practice 4 th Edition Published by. Which document created in 1964 forms the basis of ethical considerations in clinical research. We at GCP Finding are pleased to provide free GCP multiple choice questions.
National Committee for Clinical Research NCCR National Pharmaceutical Regulatory Agency NPRA-secretariat Lot 36 Jalan Universiti 46200 Petaling Jaya Selangor Malaysia. Play this game to review Education. Examination at IMU Bukit Jalil.
National Committee for Clinical Research NCCR National Pharmaceutical Regulatory Agency NPRA-secretariat Lot 36 Jalan Universiti 46200 Petaling Jaya Selangor Malaysia. And international GCP standards for drugs biologics and medical device clinical trials. It is of utmost importance.
Wednesday Thursday 830am 430pm GMT 8 DAY 3. Please be informed that the GCP examination format has been changed to an open book format. Malaysian Guideline for Good CliniCal PraCtiCetHird edition introdUCtion to MalaYSian GUideline For GCP Good Clinical Practice GCP is an international ethical and scientific quality standard for designing conducting recording and reporting clinical trials that involve the participation of human subjects.
As attached the 4th edition Malaysian Guideline for Good Clinical Practice for your reference. The revision is intended to increase the efficiency and quality of clinical trials with. Saksi yang tidak memihak didefinisikan di dalam pedoman ICH GCP sebagai pengamat penelitian independen yang merepresentasikan IRB atau Komite Etik.
The passing mark is 50. Malaysia Guideline for Good Clinical Practice 3rd Ed Type text Page 9 Introduction to Malaysian Guidelines for GCP The universal definition of Good C linical P ractice GCP is an international ethical and scientific quality standard for designing conducting recording and reporting trials that involve the participation of human subjects. Take up the quiz below and get to refresh your memory as you test your understanding.
830am 430pm GMT 8 DAY 3. Breakfast Preparation for exam. Regulatory Aspects of Clinical Trial in Malaysia.
Each question has four 4 options with a single correct response only. Good clinical practice provides a framework of principles that aim to ensure the safety of research. Once you are registered you can also post your GCP score to Leader board.
100 Multiple Choice Questions MCQ in open book format. Our online GCP trivia quizzes can be adapted to suit your requirements for taking some of the top GCP quizzes. The hard copy will be given during the examination as a reference.
The National Committee for Clinical research NCCR has commissioned the update of the Malaysian Guideline for Good Clinical Practice following the revision of International Council for Harmonisation ICH E6 Good Clinical Practice guidance in November 2016. Ministry of Health Malaysia Institute For Clinical Research Block B4 National Institutes of Health NIH No1 Jalan Setia Murni U1352 Seksyen U13 40170 Shah Alam Selangor Darul Ehsan Malaysia Phone. The 4th edition Malaysian Guideline for Good Clinical Practice is also available at.
Share free summaries past exams lecture notes solutions and more. Up to 12 cash back In this course we will learn what Good clinical practices are guidelines of what an ethical and safe trial is the rights and importance of consent of the trial subjects the duties of the sponsor and investigator of the trial and much more. This is a really good way to both expand and test your knowledge of GCP.
Overview of GCP and Clinical Research in Malaysia. Good Clinical Practice Quizzes- SET 1. 03-7883 5400 Homepage.
Hong Kong together with the registration form to Clinical Trials Centre6F Block T Queen Mary Hospital 102 Pokfulam Road Hong Kong Attention. This exam contains negative marking. A Question Answer Reference Guide 20202021 Electronic This industry-leading GCP reference guide answers over 1000 of the most common and difficult questions regarding the interpretation and implementation of US.
Good Clinical Practice Examination. GCP Compared to Malaysian Guideline for GCP. Any individual member of the clinical trial team designated and supervised by the investigator at a trial site to perform critical trial-related procedures andor to make important trial-related decisions eg.
Associates residents research fellows. Malaysian Guideline for Good Clinical Practice 4th Ed Malaysian Guideline for Good Clinical Practice 4th Edition Published by. Since the inception of the Clinical Research Centre by Ministry of Health in August 2000 Good Clinical Practice GCP has become an important aspect in the conduct of clinical trials in Malaysia.
Physicians researchers and healthcare. All questions shall be answered within 3 hours and 30 minutes. JOIN OUR GOOD CLINICAL PRACTICE GCP WORKSHOP.
It is of utmost importance that this standard is. International Council of Harmonization ICH.

Ctn Webinar Good Clinical Practice Gcp Refresher And Trends In The Ctn Youtube

Good Clinical Practice Gcp Lecture 1 Introduction Principles Of Gcp Eventtroop Youtube

Introduction To Clinical Research Ii

Good Clinical Practice Gcp Lecture 1 Introduction Principles Of Gcp Eventtroop Youtube

Pdf Clinical Practice Guidelines For The Management Of Non Specific Low Back Pain In Primary Care An Updated Overview

Clinical Practice Guideline Benign Paroxysmal Positional Vertigo Update American Academy Of Otolaryngology Head And Neck Surgery Aao Hns

Good Clinical Practice Gcp Lecture 1 Introduction Principles Of Gcp Eventtroop Youtube
Clinical Practice Guidelines And Consensus Statements For Antenatal Oral Healthcare An Assessment Of Their Methodological Quality And Content Of Recommendations Plos One

Clinical Research Associate Resume Samples Velvet Jobs
(103).jpg)
Good Clinical Practice Quiz Questions And Answers Proprofs Quiz

Clinical Research Associate Resume Samples Velvet Jobs
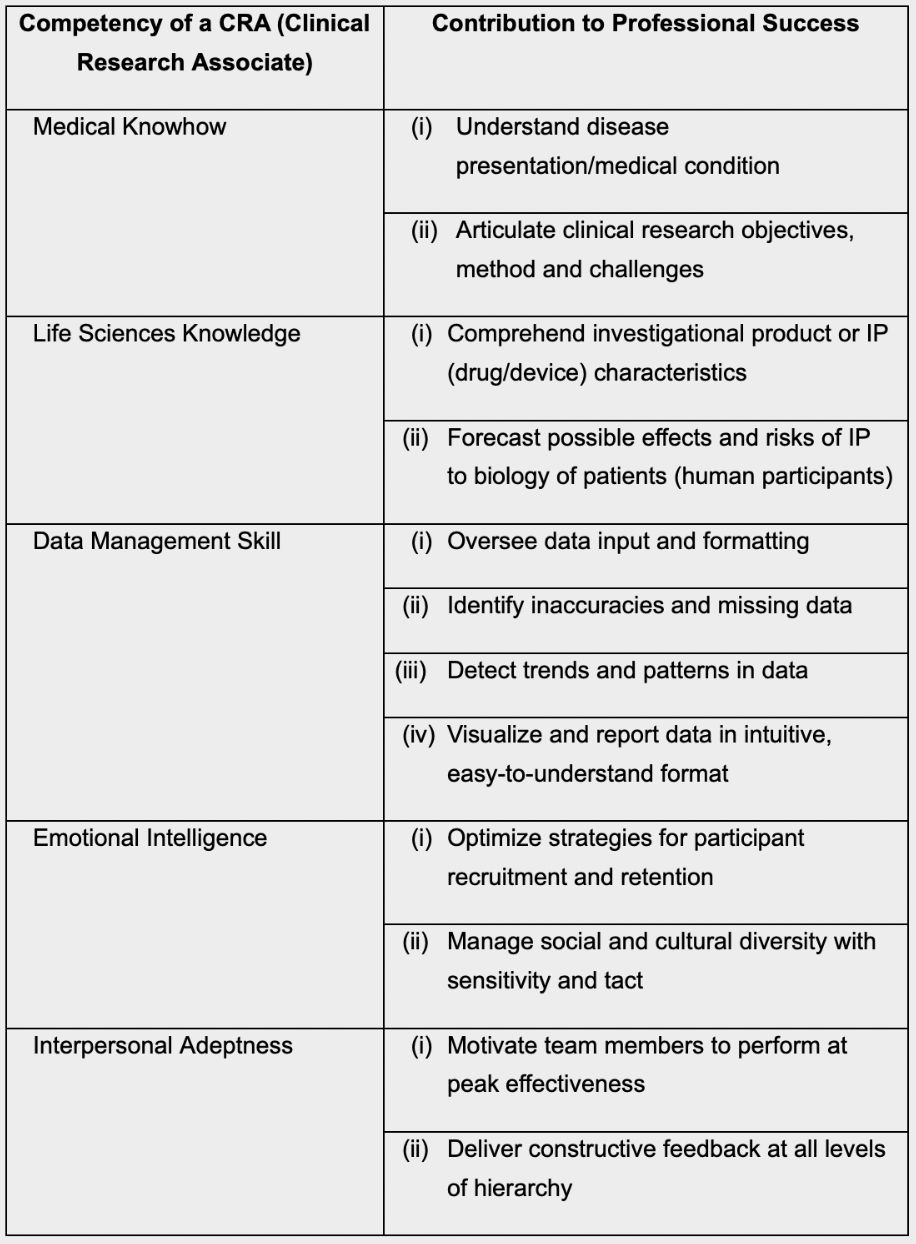
Clinical Research Associate Cra A Complete Guide On How To Become A Clinical Research Associate Clinical Research Certification

Clinical Research Associate Resume Samples Velvet Jobs
Clinical Research Associate Cra A Complete Guide On How To Become A Clinical Research Associate Clinical Research Certification

3 Examples Of Nursing Best Practices Uri Online

Good Clinical Practice Gcp Lecture 1 Introduction Principles Of Gcp Eventtroop Youtube



